Nucleic acid drugs - Q&A section
release date:2023-03-16 view count:2361
1. What are nucleotides, nucleosides, and nucleic acids?
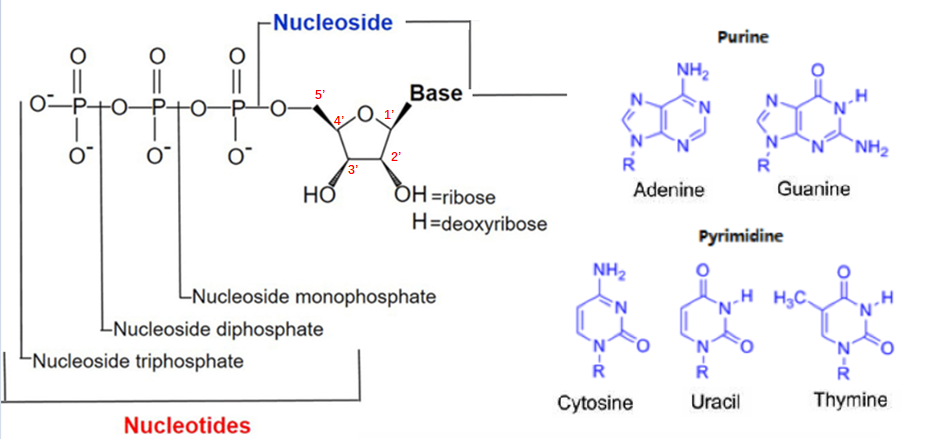
·Nucleotides are composed of a nitrogen-containing base, a five carbon sugar, and a phosphate acid molecule.
According to the structure of the mother nucleus, the base is divided into two categories: purine and pyrimidine, with purines including adenine and guanine; Pyrimidine includes cytosine, thymine, and uracil.
§ There are two kinds of ribose: Ribose and deoxyribose. Nucleotides containing ribose are called ribonucleotide, which is the basic unit of RNA; Nucleotides containing deoxyribose are called deoxyribonucleotides, which are the basic units of DNA.
·Nucleoside refers to the removal of phosphate molecules from nucleotides. According to the number of phosphate molecules, nucleotides can be divided into nucleoside monophosphate, nucleoside diphosphate, and nucleoside triphosphate.
·Nucleic acid is the genetic material of humans, which is a polymer composed of nucleotides connected by 3 ', 5' - phosphate diester bonds.
2. What is a Nucleic Acid Drug? Nucleic acid drugs refer to drugs developed by utilizing the translation or regulatory functions of nucleic acid molecules, which exert pharmacological effects through various splicing modifications and carriers of nucleotide chains. Small molecule and antibody drugs use "proteins" as drug targets, and many "pathogenic proteins" in the human body are non drug targets, thus many diseases lack effective treatment methods. Nucleic acid drugs are expected to address the unmet clinical needs brought about by "non producible" targets, and will become the third main class of drugs after small molecule and antibody drugs. It can be divided into:
DNA based therapy, known as gene therapy, includes in vivo gene therapy and in vitro gene therapy (cell therapy). At present, this technology is relatively mature, and dozens of drugs have been launched.
RNA based drugs, known as RNA drugs, are classified according to their mechanisms and functions (including but not limited to): aimed at encoding proteins, such as mRNA; With nucleic acid (DNA or RNA) as the target, such as ASO, or RNA interference (siRNA or miRNA); Targeting the three-dimensional structure of proteins as aptamer drugs.
3. What is oligonucleotides? Oligonucleotides usually refer to DNA or RNA fragments synthesized in the laboratory, which are short stranded nucleic acids composed of tens to tens of nucleotides in series. Oligonucleotides are widely used in biochemistry, biology, medical molecular testing, genomics, and other molecular biology experiments. In biochemical experiments, oligonucleotides have two main uses:
Primers primer: A small segment of DNA or RNA used for PCR amplification, serving as the starting point for nucleotide chain extension during amplification.
Probes probe: used to label amplification products, with detectable fluorescent molecules, capable of specifically binding to amplification products, emitting fluorescence signals that are detected by the instrument.
4. What are oligonucleotide drugs?
Oligonucleotide drugs, also known as small nucleic acid drugs, are short stranded nucleic acids composed of tens to tens of nucleotides in series. They act on pre mRNA or mRNA and achieve disease treatment goals by interfering with target gene expression. At present, small nucleic acid drugs mainly include siRNA drugs (small interfering RNA) and ASO drugs (antisense oligonucleotides).
The opposite of short stranded small nucleic acid drugs is long stranded nucleotide drugs (mRNA drugs).
5. What is chemically modified nucleoside? Oligonucleotide drugs without chemical modification are usually not ideal, and they have poor PK characteristics, such as poor stability, easy to be degraded by nuclease; Large polarity, difficult to enter cells, poor distribution characteristics; Poor binding affinity to target RNA. In order to achieve clinical efficacy, oligonucleotides must undergo chemical modification.
Classification by modification site: Different site modifications can produce different pharmacological effects
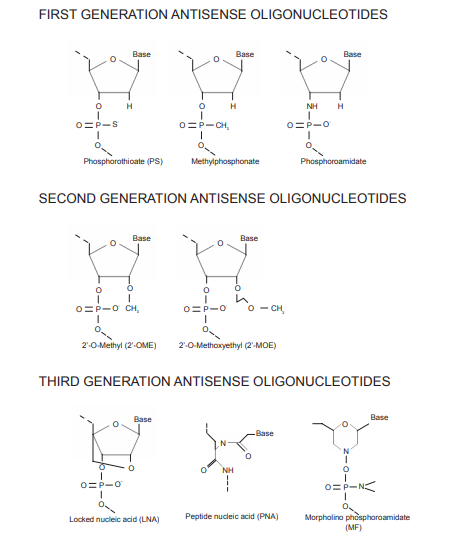
► Backbone modifications: The most common chemical modification of the phosphate skeleton is thiophosphoric acid, that is, a non bridging oxygen in the phosphate skeleton in nucleotides is replaced by sulfur. PS modification basically does not affect the activity of nucleic acid drugs, but can resist the degradation of nuclease. Moreover, this modification can enhance its binding ability with plasma proteins, reduce the renal clearance rate, and improve the half-life. PS is a common chemical modification in the first generation ASO drugs and is still frequently used in nucleic acid drugs. But after PS modification, the affinity between nucleic acid drugs and complementary nucleotide chains will decrease, so subsequent chemical modifications are needed to improve.
► Ribose: The second generation of chemical modification mainly focuses on the structure of ribose, which modifies the 2-position hydroxyl/hydrogen in the ribose structure. Common modifications include 2 '- MOE (less used in siRNA), 2' - OMe, and 2 '- F. These modifications can further enhance the resistance to nuclease and enhance its binding ability with complementary nucleotide chains.
► Transformation of the five membered ring of ribose: transformation of the five membered ring of ribose is called the third generation of chemical modification, including LNA (locked nuclear acid), PNA (peptide nuclear acid), PMO (phosphoramidate morpholino oligomer), which can further enhance the resistance of nucleic acid drugs to nuclease, improve the affinity and specificity. Because PNA and PMO are charge neutral, their binding ability with plasma protein is weak, so they are easy to be cleared and have a short half-life. However, they can covalently bind with some groups to improve the efficiency of nucleic acid drugs entering cells, including lipids, peptides, aptamers, antibodies, GalNAc, etc.
Base: Nucleic acid drugs have poor tolerance to base modification, but base modification is also being attempted, such as cytosine methylation, which can increase its melting temperature. It has been applied in the design of ASO drugs.
► End modification: To prevent the nucleotide chain from being degraded by nucleic acid exonucleases, it is necessary to protect the end of the nucleotide chain, including adding trans thymidine at the 3 'end, or adding palmitic acid or coupling aromatic compounds at the end.
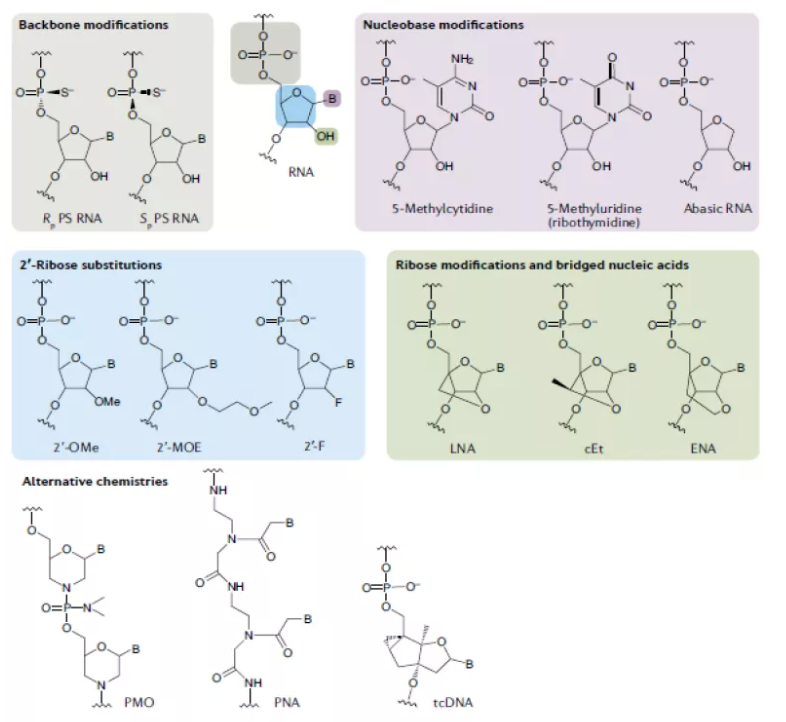
The development process can be divided into three generations: after decades of accumulation, the chemical modification of oligonucleotides has developed three generations of technology. The commonly used chemical modifications are the first generation thiophosphates and the second generation methyl phosphates, which are uncharged and therefore more lipophilic than natural DNA or RNA, and can penetrate cells better. Subsequently, third-generation PNA, LNA and other technologies emerged.
6. What are Protected Nucleosides? In the artificial synthesis process of oligonucleotide synthesis, in order to prevent unnecessary side reactions, all other functional groups in the nucleoside must be protected by attaching a protective group. The efficiency of oligonucleotide synthesis largely depends on the protective groups on the nucleotide phosphoramide, which must be carefully selected and selectively incorporated into different positions, such as the outer ring amino group on the base and the 5 'and 2' positions of the hydroxyl group. After the assembly of the oligonucleotide chain, all protective groups are removed to produce the desired oligonucleotide.
7. What is Phosphoramidites? Phosphosamide is a specific protective nucleoside, which is currently the standard compound for oligonucleotide synthesis. It can sequentially add new bases to the DNA strand in an extremely simple and effective cyclic reaction. Compared with previous protective nucleotides, the synthesis efficiency is higher and there are fewer by-products.

The above figure shows the structure of a standard 2 '- deoxyadenosine phosphoramide.
Green: The dimethyltrimethyl (DMT) group that protects the 5 'hydroxy group on deoxyribose is easily cleaved under acidic conditions.
Yellow: In the presence of an azole catalyst, diisopropylamino removes phosphoramide and growing oligonucleotides in an almost instantaneous reaction.
Orange: A type of 2-cyanoethyl that protects the second hydroxyl group on phosphite and is easily removed under mild alkaline conditions.
Pink: Protects variable groups of amino groups in nitrogen-containing bases, such as 2-cyanoethyl, which are ultimately removed under alkaline conditions.




