Overview of the Small Nucleic Acid Drug Market
release date:2023-03-27 view count:2442
Recently, GSK announced that it would abandon the development of cell and gene therapies and switch to small nucleic acid drugs. Small nucleic acid drugs, also known as oligonucleotide therapies, are a class of single or double stranded small nucleotides with a dose of about 20 milliliters. They are currently one of the most mature gene therapies and are expected to become the third class of drugs after small molecule and antibody drugs.
Classification of small nuclear acid drugs
At present, small nucleotide drugs include antisense oligonucleotide (ASO), small interfering RNA (siRNA), microRNA (miRNA) and aptamer.
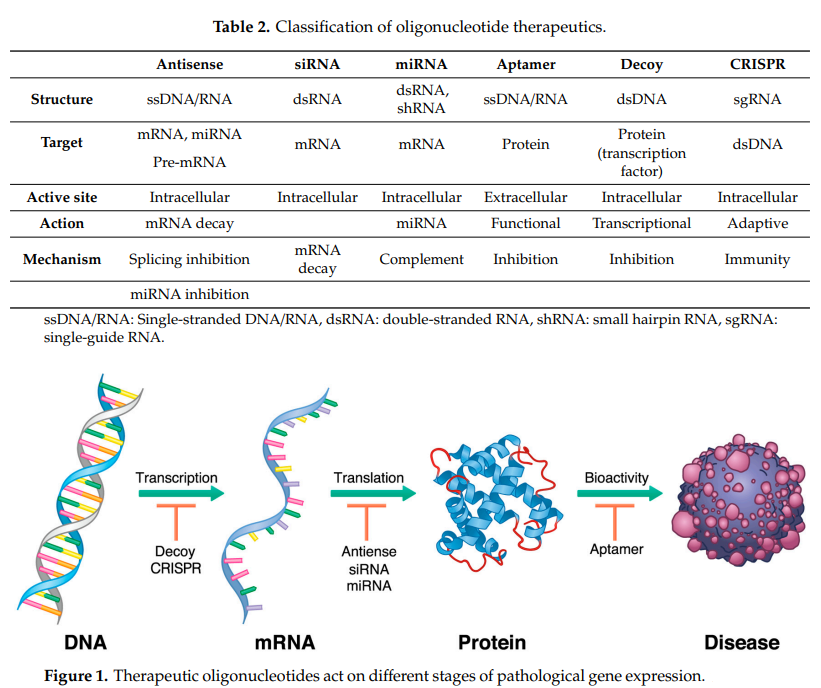
已获批和在研的小核酸药物以ASO和siRNA为主。
[4]
Antisense oligonucleotide (ASO)
ASO is a single stranded nucleotide composed of approximately 18-30 nucleotides, typically complementary to mRNA sequences, primarily by binding to the target RNA and silencing the target gene.
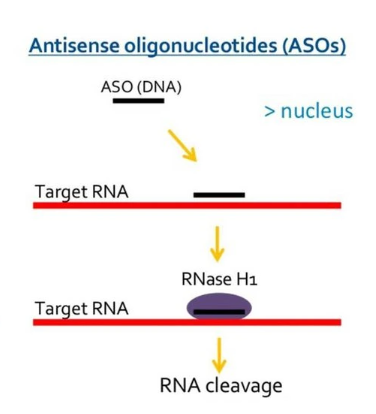
According to the mode of action, it can be divided into two categories: gene expression inhibitors and splicing regulators:
·The expression inhibitor works through RNAse H, which binds to the DNA/RNA double strand and degrades the RNA strand.
·Splicing regulators bind to the intron exon junction of pre mRNA, inducing steric hindrance to prevent splicing events.
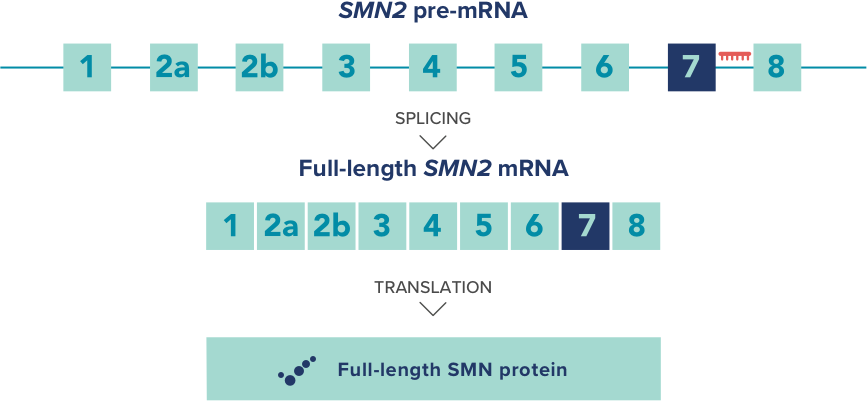
The representative drug in splicing regulators is Spinraza, which is used to treat spinal muscular atrophy (SMA). Spinraza targets the specific sequence of the downstream intron of SMN2 exon 7 to alter alternative splicing and induce exon 7 to remain in the transcript. It is a fully modified PS, 2 '- MOE ASO, administered intravenously every two weeks.
siRNA
SiRNA molecules are effector molecules of RNAi, typically composed of 19+2 mer structures (i.e. 3 of 19 complementary bases and 2 terminal nucleotides) ʹ Cantilever).
The main principle is that when siRNA double strands are fed into cells, they are assembled into RNA induced silencing complexes (RISCs). RISCs remove one of the messenger strands and guide the other strand to pair RISCs with mRNA, cutting unnecessary target mRNA and degrading it, thereby silencing specific genes related to the disease, achieving therapeutic purposes.
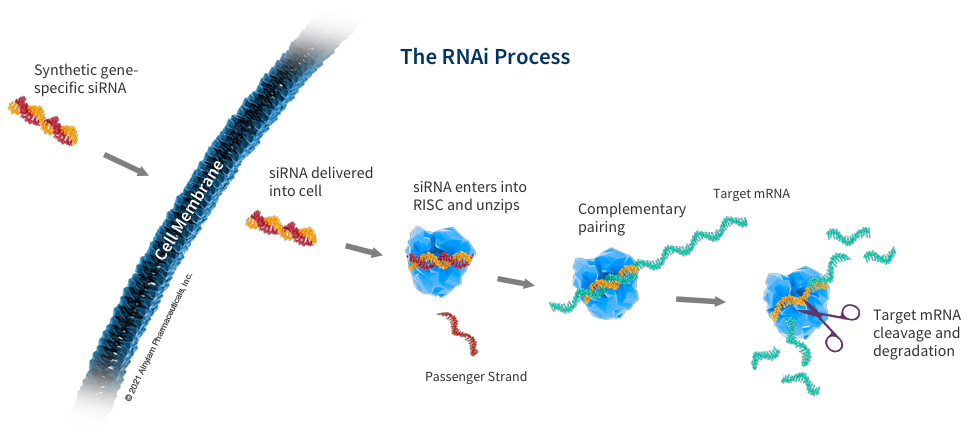
The main advantage of small nuclear acid drugs is that compared to small molecule or antibody drugs, they can enter the cell and act on upstream mRNA, with high specificity and can target specific genes; Not limited by target drug formation; Development is relatively simple and has a high success rate; Long half-life and low administration frequency; Fast production speed, etc.
Delivery technology
One of the challenges of small nuclear acid drugs is the delivery problem, and currently the main delivery tissues are the liver, CNS, and eyes. Due to the development of LNP and GalNAc, the convenience of targeting the liver has been promoted. Therefore, currently, most targeted tissues are mainly focused on the liver, while the injection methods for LNP and GalNAc are mainly intravenous and subcutaneous injection. The second largest target tissue is CNS, which cannot reach the central nervous system after systemic administration such as subcutaneous or intravenous injection due to the blood-brain barrier. Therefore, the administration method for CNS is mainly intrathecal injection.
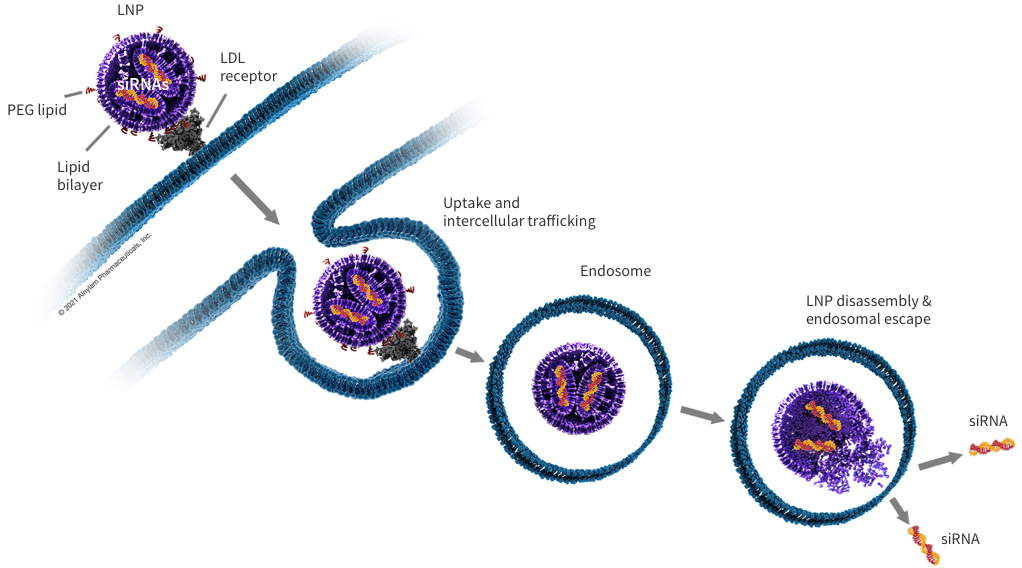
Lipid nanoparticles (LNPs)
LNPs are chemically synthesized multi-component lipid formulations (approximately 100 nanometers in size) that encapsulate siRNAs for delivery to target tissues.
LNPs are delivered by intravenous injection (IV). During delivery, siRNA encapsulated in LNPs is protected from degradation by nuclease. The LNPs used by Alnylam are preferentially assigned to the liver because they have affinity with lipoprotein E (apoE).
Coupled delivery
Couplings can bind fully modified siRNAs to targeted ligands and deliver them to specific cells or tissues in the body. Alnylam has developed two conjugates for targeted transmission of liver and central nervous system (CNS) - GalNAc conjugates and CNS targeted siRNA conjugates.
GalNAc (N-acetyl galactose amine) is a trivalent sugar molecule. It is gathered by three GalNAcs and can recognize ASGPR receptors on the surface of liver cells. Compared with a single GalNAc, its affinity is significantly enhanced and its delivery efficiency is higher.
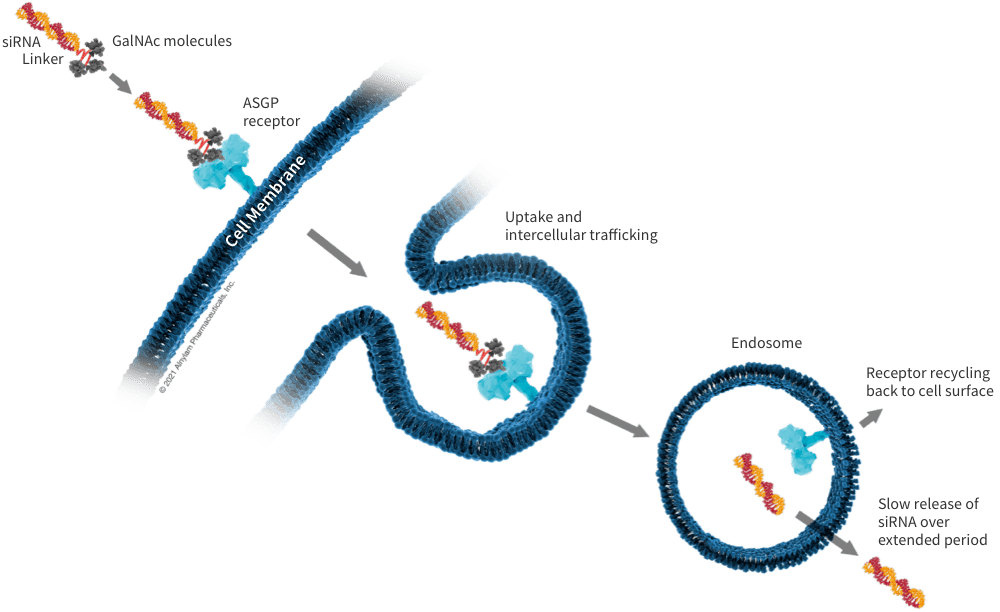
GalNAc delivery therapy is achieved through subcutaneous injection. Currently, except for ONPATTRO, which is delivered through LNP, most of the marketed and currently under research pipeline drugs are delivered through GalNAc.
market size
In 1978, Harvard University's Zamecnik et al. first proposed the concept of antisense nucleic acid. In 1998, the first small nucleic acid drug Vitravene was approved for use in the treatment of cytomegalovirus retinitis. However, due to the development of highly active antiretroviral therapy, the number of cytomegalovirus cases sharply decreased and was delisted due to low sales. The three drugs approved for marketing in the early stages (1998-2013), Vitravene, Macugen, and Kynamro, have all been delisted. In the past five years, with the increase in approval of small nuclear acid drugs, the industry has begun to accelerate its development.
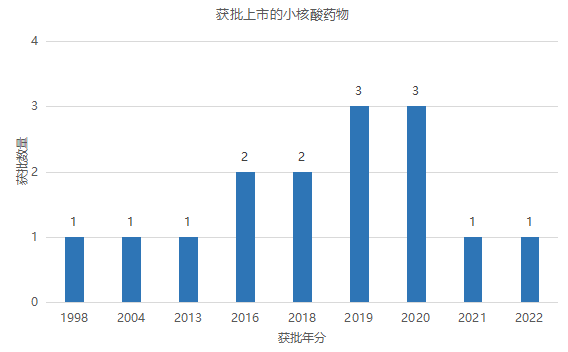
The global small nucleic acid drug market size in 2022 is approximately 3.8 billion US dollars, with Ionis/Biogen's Spinraza becoming a blockbuster, with global sales of 1.794 billion US dollars in 2022.
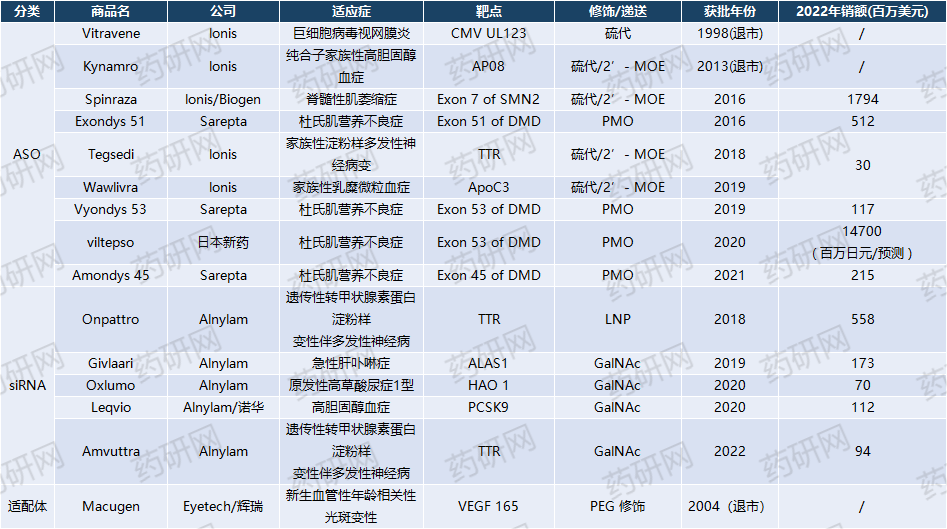
Compared to foreign countries, only Spinraza drug has been approved in China, which was approved for marketing in 2019 and entered medical insurance in 2021.
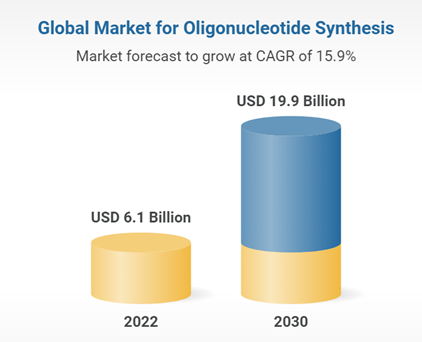
According to a Research and Markets report, the market size of oligonucleotide drugs will reach $8.6 billion by 2030. The global oligonucleotide synthesis market is expected to reach $19.9 billion by 2030, with the Chinese market reaching $4.9 billion, with a compound annual growth rate of 21.5%.
Under research pattern
According to Frost&Sullivan's statistics, there are currently nearly 108 small nucleic acid drugs entering clinical practice worldwide, mostly in the early clinical stage. According to the public data of Smart Bud, there are still more than 300 drugs in the preclinical stage worldwide.
At present, the small nuclear acid drugs under research are mainly ASO and siRNA, and the indications with more layouts are tumors (24%), genetic diseases (22%), sensory organ diseases (13%), etc.
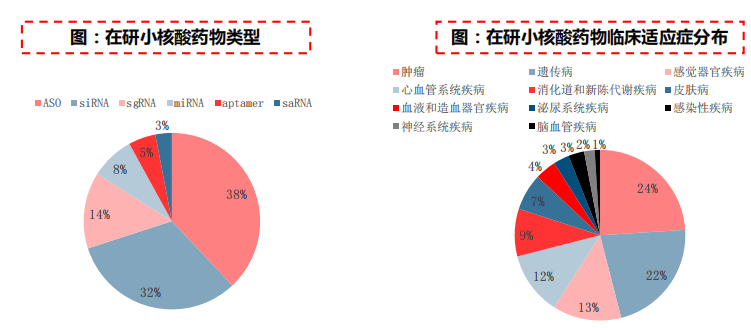
Source: Frost&Sullivan, China CITIC Construction Investment Corporation
Domestically speaking, multiple drugs have entered the late clinical stage, among which Novartis' Incisiran and Japanese new drug Viltolarsen have submitted marketing applications.
data
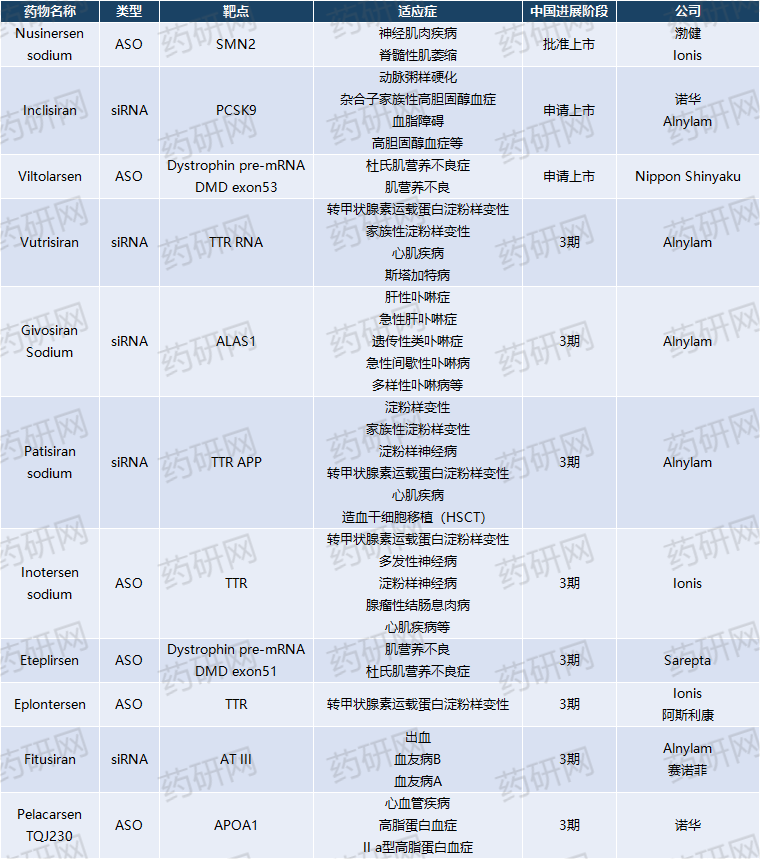
In summary, small nuclear acid drugs still face challenges in delivery technology, production, and other aspects. However, compared to small molecule and antibody drugs, small nuclear acid drugs have great potential in the field of "non producible" diseases.




