Interpretation of Full Knowledge Points on Method Validation
release date:2023-03-27 view count:2308
Due to the change of the name of method validation to method validation in the latest version of CNAS-CL01:2018 "Accreditation Criteria for Testing and Calibration Laboratory Capability", we will replace method validation with method validation.
1、 Definition
Method confirmation is the process of confirming whether non-standard methods, laboratory developed methods, standard methods used beyond the predetermined scope, or other modified standard methods can be reasonably and legally used.
Method validation refers to the process of evaluating the laboratory's ability to carry out testing and calibration activities in terms of human, machine, material, method, environment, and measurement before introducing standard or non-standard methods into the laboratory.
2、 Differences
1. Different objects
1) The objects of method confirmation are non-standard methods, including some non-standard methods, laboratory developed methods, standard methods used beyond the predetermined scope, and other modified standard methods.
2) The objects of method validation are standard methods and confirmed non-standard methods.
2. Different purposes
1) The purpose of method confirmation is to confirm whether the non-standard method can be used reasonably and legally.
2). The purpose of method validation is to verify whether the laboratory has the ability to carry out testing/calibration activities according to the method requirements.
3) To understand the difference between the two, one must first understand their definition.
Method validation, referred to as "verification" in English standards, can be translated as "confirmation" or "validation". In laboratory work, it is defined as "providing objective evidence to prove that a certain project meets the specified requirements".
Method confirmation, referred to as "validation" in English standards, can be translated as "confirmation". Its definition is "the determination that a specific intended use or application requirement is met by providing objective evidence
The two are very similar in definition, and in practical operation, validation or confirmation parameters also need to be evaluated from parameters such as detection limit, quantification limit, sensitivity, selectivity, linear range, measurement range, etc.
From this perspective, method validation and method validation have the same goal from a technical perspective, but in reality, there are fundamental differences between the two. The most crucial difference is that the objects of method validation and method validation are different.
Method validation is conducted against standard methods. According to "17025", before introducing testing or calibration, the laboratory should verify that these standard methods can be correctly applied. Standard methods refer to technical specification documents publicly released by recognized institutions after evaluation and confirmation. Before using standard methods, laboratories should undergo method validation, also known as method validation, which means that before using standard methods for testing, the laboratory needs to verify whether its technical ability to use standard methods meets the specified requirements of the standard.
Method confirmation mainly targets non-standard methods, and their objects are specified in the "17025" standard. From a broad understanding, all testing methods except for those specified in international, regional, national, industry, and local standards belong to non-standard methods. Except for the first type of methods that belong to standard methods, the other three types belong to non-standard methods.
When the testing method used in the laboratory is a non-standard method, it is necessary to confirm the non-standard method and differentiate and discuss the different types of non-standard methods.
01- Non standard methods recognized by the government or recognized institutions
Technical methods issued by relevant national or industry authorities, although not standard methods, can be directly used as they have been recognized by authoritative institutions and do not require further confirmation. However, before being put into use, the laboratory needs to verify to ensure that it has the ability to implement these methods.
02- Methods for publishing well-known technical organizations or related scientific books and journals
Renowned technical organizations such as certain companies and industry associations that are widely adopted internationally and widely recognized in the industry, whose standards require recognition from industry regulatory authorities in certain specific industries to prevent inconsistency with regulatory regulations, can also be directly used by laboratories after capability verification.
03- Method developed by equipment manufacturer
When using the method developed by the equipment manufacturer, the laboratory should make a judgment based on the type of test sample. If the test sample belongs to the type specified in the instrument equipment method and the value is within the range of instrument measurement, the laboratory can directly confirm and use the method. Otherwise, the laboratory also needs to confirm the method, conduct technical confirmation, and develop a document.
04- Method designed and developed by the laboratory itself
The methods designed and developed by the laboratory themselves, or the standard methods used beyond the predetermined scope, as well as the expanded and modified standard methods, must undergo technical confirmation. The confirmation can be conducted by industry experts, and proof materials issued by authoritative technical institutions that the method is accurate and reliable must be provided. If the laboratory itself is an authoritative institution in the industry, it can organize technical confirmation by the laboratory itself, and if necessary, provide external proof materials.
3、 Overall principles of method validation
When introducing standard methods in a chemical analysis laboratory, the laboratory should verify whether the operation of the method meets the requirements of the standard based on quantitative and qualitative analysis, that is, to confirm that the method can achieve satisfactory results under the existing facilities, equipment, personnel, environment, and other conditions of the laboratory. If necessary, the laboratory can participate in capacity verification or conduct inter laboratory comparisons.
If only minor modifications are made to the standard method (as long as they are made), such as using similar equipment or reagents from different manufacturers, validation should also be conducted if necessary to prove that satisfactory results can be obtained, and the modified content should be formulated as a work instruction document.
1. Validation of quantitative analysis methods
The key parameters in the method validation process should depend on the characteristics of the method and the detection range of the sample matrix that may be measured, at least measuring accuracy and precision. For trace chemical analysis laboratories, they should also ensure that appropriate LOD and LOQ are obtained. In general, the parameter selection for quantitative analysis method validation can refer to the table below.
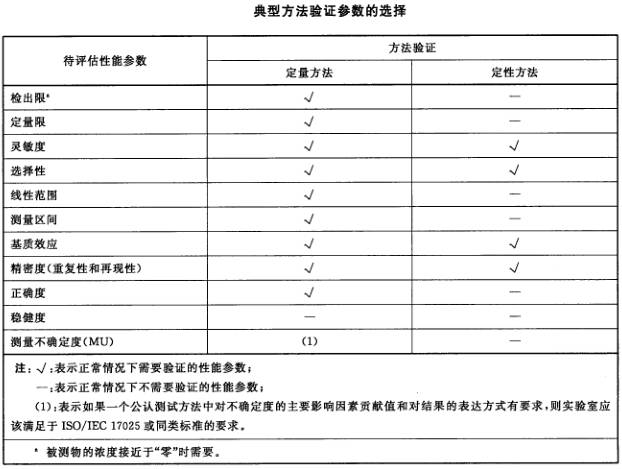
2. Qualitative analysis and validation of qualitative methods
The precision of analysis is usually expressed as the false positive or false negative rate, which can be determined using different concentration levels.
Laboratory method validation can analyze a set of negative or positive reinforcement samples. For each different sample matrix, two duplicate samples are analyzed at three levels of content.
The recommended content levels are blank (without analytes), low concentration (close to the lowest applicable concentration of the method), and high concentration (close to the highest applicable concentration of the method). The standard addition method can be used to obtain appropriate concentrations, that is, when testing according to the same method, the test sample is divided into two or more parts. Some parts are subjected to routine analysis, while others are added with known amounts of standard analytes before analysis.
The amount of standard analyte added should be 2 to 5 times the estimated content of the analyte in the sample, or it can be the amount of standard analyte calculated according to the quantitative limit, allowable limit, etc. of the method.
The standard addition method can be used to measure the recovery rate of the method, which may be influenced by factors such as the content of analytes and matrix in the measured sample, and can also be used to evaluate the accuracy at levels such as quantification limit and tolerance limit. The false positive or false negative rate should be comparable to the data confirmed by the method in order to demonstrate the laboratory's ability to use the method. In general, the parameter selection for qualitative analysis method validation can refer to the table above.
4、 How does the laboratory conduct method validation?
1. General requirements for laboratory method validation:
1) The standard preparation team shall prepare the method validation scheme, and select the appropriate laboratory, sample type, content level, analysts, analytical equipment, analysis time and other contents according to the main factors affecting the precision and accuracy of the method and the requirements of mathematical statistics.
2) The standard preparation team should not only use certified reference materials/standard samples, but also provide actual samples for method validation. The actual samples should cover the scope of application of the method standard as much as possible.
3) Before method validation, the operators participating in the validation should be familiar with and master the principle, steps, and processes of the method, and receive training if necessary.
4) The reagents, materials, instruments, equipment, and analytical steps used in the method validation process should comply with the relevant requirements of the method.
5) The operators and standard preparation team participating in the validation should truthfully fill out the "Original Test Data Table" in the "Method Validation Report" according to the requirements. If necessary, graphs or other record printing strips generated by the instrument that match the content of the original test data table should be attached.
6) The standard preparation team will form a "Method Validation Report" based on method validation data and statistical, analytical, and evaluation results.
2. There are 6 methods for confirming methods:
1) Use reference standards or reference materials for calibration or evaluation of bias and precision.
2) Conduct a systematic review of the factors that affect the results.
3) Test the robustness of the method by changing the control parameters, such as the temperature of the incubator, the sample volume, etc.
4) Compare the results with other confirmed methods
5) Interlaboratory comparison
6) Evaluate the uncertainty of measurement results based on an understanding of the principles of the method and practical experience in sampling or testing methods.
3. The method validation should include the following aspects:
1) An evaluation of the human resources required to implement the new method, that is, whether the testing/calibration personnel have the necessary skills and abilities, and if necessary, personnel training should be conducted before taking up the job after assessment.
2) Is it necessary to supplement new standards or reference materials for the evaluation of the applicability of existing equipment.
3) Evaluation of whether the preparation of items, including pre-treatment, storage, and other aspects meet the requirements of the new method.
4) Evaluation of operational standards, uncertainty, original records, report formats, and their suitability for new method requirements.
5) Evaluate the facilities and environmental conditions, and verify if necessary.
6) The evaluation of the correct application of new methods, when there are changes to old methods, should be compared between the new and old methods, especially the evaluation of difference analysis and comparison.
7) Perform two or more complete simulation tests/calibrations according to the new method requirements, and issue two complete result reports.
Note: 1. Method confirmation should be documented and recorded accordingly. When modifying methods that have already been confirmed, the impact of these modifications should be determined. If it affects the original confirmation, it should be re confirmed.
2. Method validation should be documented and recorded accordingly. When the method changes, it should be re validated.
5、 Specific requirements for laboratory method validation
1. Verification of detection limit
Determine the detection limit, analyze and operate according to the steps and procedures of the method, calculate the average value, standard deviation, relative standard deviation, detection limit, and other parameters of the results. The final method detection limit is the highest value of the data obtained from each validation laboratory.
2. Verification of precision
Determination of certified reference materials/standard samples: A unified sample with three different content levels (including a concentration or content near the lower limit of measurement) is used, and each sample is measured more than 6 times in parallel. The average value, standard deviation, relative standard deviation, and other parameters of samples with different concentrations or contents are calculated separately.
Measurement of actual samples: Each validation laboratory should analyze and test samples of the same type with 1-3 levels of content. Each sample should be tested more than 6 times in parallel, and the average value, standard deviation, relative standard deviation, and other parameters of different samples should be calculated separately.
Summarize and analyze the data of each validation laboratory, calculate the relative standard deviation, repeatability limit r, and reproducibility limit R between laboratories.
3. Verification of accuracy
If each validation laboratory uses certified reference materials/standard samples for analysis and determination to determine accuracy, it is necessary to measure 1-3 certified reference materials/standard samples with different content levels. Each certified reference material/standard sample should be tested in parallel for more than 6 times according to the entire program, and the average value, standard deviation, relative error, and other parameters of certified reference materials/standard samples with different concentrations or content levels should be calculated separately.
If the laboratory conducts spiking analysis on actual samples to determine accuracy, it is necessary to add a certain amount of certified reference materials/standard samples to 1-3 unified samples with different content levels for each sample type for measurement,
Each spiked sample shall be tested in parallel for more than 6 times, and the spiked recovery rate of each unified sample shall be calculated separately.
Summarize and analyze the data of each validation laboratory, calculate the mean and range of relative error or spiked recovery rate.




